Measuring & Analyzing Greenhouse Gases: Behind the Scenes
Greenhouse Gases and Radiation
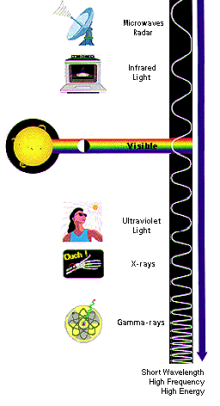
So you may be wondering…what makes a gas a greenhouse gas?
The sun is a burning hot ball of gas (surface temperature of about 5,500 °C or 10,000 °F!) that emits a wide range of radiation. Some of the sun’s radiation is ultra-violet, the kind of radiation that gives you a sunburn. Some of the radiation from the sun is in the visible light spectrum, meaning that you can see it. Finally, some of the radiation emitted by the sun is infrared radiation, a type of longwave radiation. Other forms of longwave radiation that you might be more familiar with include heat lamps, fires, microwaves and radiowaves.
When radiation from the sun enters the Earth's atmosphere, some is absorbed by the atmosphere, some is reflected by clouds and by the surface of the Earth straight back into space, and some is absorbed by the surface of the Earth. If the Earth simply kept absorbing incoming radiation and without emitting any of its own, it would get hotter and hotter through time. Fortunately, the surface of the Earth also emits radiation. No matter what the incoming radiation wavelength is, the Earth emits the radiation back as infrared radiation.
What is infrared radiation?
Everything that has temperature emits infrared radiation. This includes the Earth. Some of the infrared radiation escapes into space, but some is stopped and absorbed by greenhouse gases in the atmosphere. Just like things that have temperature emit infrared radiation and cool as a result, things that absorb infrared radiation increase in temperature. So, the greenhouse gases increase in temperature, and they share that heat with the rest of the molecules in the air. Now, the warmer greenhouse gases emit infrared radiation based on their temperature.
When the Earth emits radiation, it has nowhere to go but up, but greenhouse gases can emit radiation in all directions. Some radiation escapes to outer space, some is reabsorbed by other greenhouse gases, and some is directed back down to the Earth. Then, that radiation can get absorbed again, and then re-emitted, and then... In short, the absorption of infrared radiation by greenhouse gases contributes to an increase in temperature of the surface of the earth and the atmosphere.
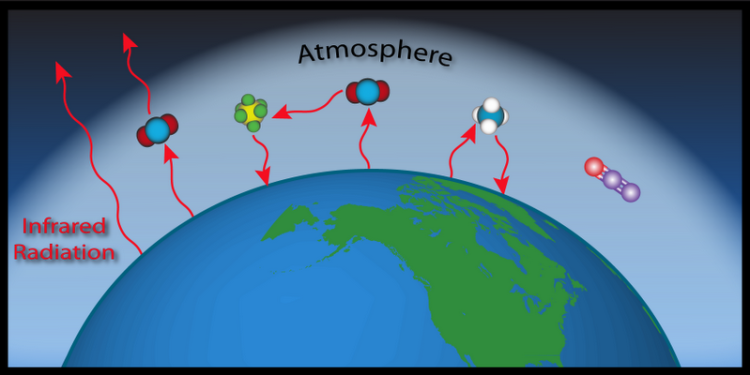
Did you ever wonder why these gasses are called greenhouse gases? They act like a giant greenhouse, allowing sunlight to get in and warm the Earth without letting all of the heat (infrared radiation) escape. If you are interested in learning more about the greenhouse effect please read about the basics of the carbon cycle.
What Makes an ‘Effective’ Greenhouse Gas?
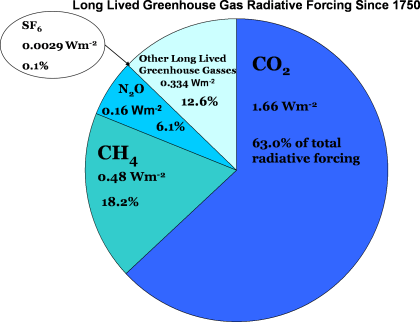
Not all greenhouse gases are created equal, and some are better at absorbing infrared radiation than others. The amount of the gas in the atmosphere is also important – the more of a gas in the atmosphere, the more effective it is. Another key feature of an effective greenhouse gas is the length of time that it stays in the atmosphere, its “lifetime”. The longer a gas is in the atmosphere, the longer it is able to continue to affect the climate system.
We use a term called “radiative forcing” to combine all of these factors into a single measure of the effect of a greenhouse gas on the Earth’s temperature. A gas that is very good at absorbing infrared radiation and warming the atmosphere has a higher radiative forcing than a gas which does not absorb very much radiation. A large amount of gas that absorbs infrared radiation also increases the radiative forcing of that gas over time.
So…What are the 3 key factors to an effective greenhouse gas?
- High radiative forcing
- Long lifetime
- A lot of it
Why do we want to measure the 6 gases we do?

 Previous
Previous