Measuring & Analyzing Greenhouse Gases: Behind the Scenes
What is the Manometric System?
The Manometric system determines the concentration of CO2 in Primary standards by first measuring the total amount of air in the sample and then separating out and measuring the CO2. Both measurements are made by using the Ideal Gas Law, which tells us that the pressure inside a volume is proportional to the amount of gas. The CCGG group measures CO2 concentrations in the Primaries with the Manometric system every couple of years in order to keep track of any "drift" (changes in concentration) that may occur over time and to narrow the uncertainty of CO2 concentrations within each Primary.
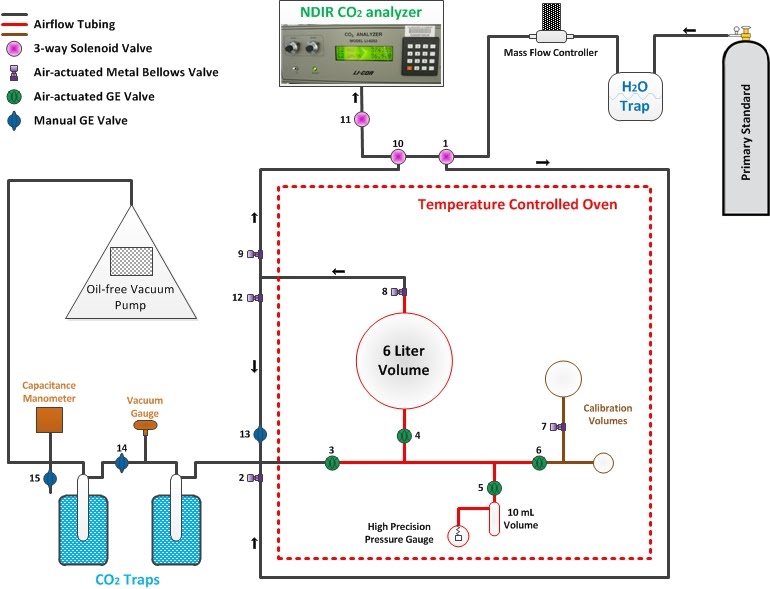
The process of measuring absolute concentrations of CO2 in Primaries is described step-by-step below:
-
Tubing Evacuated
First, all outside air is removed from the system using a vacuum pump. Then that same vacuum pump is used to pull a sample of air from the Primary tank through the system to where it needs to go. It is important to remove any room air that could contaminate the air from the Primaries.
-
Water Trapped
The air sample from the Primary standard is passed through a water (H2O) trap that contains an alcohol bath at -70ºC (-94ºF). This low temperature causes any water that is present in the air sample to freeze out on the side of the tubing.
-
6L Glass Volume Filled
The air then flows into the large 6L glass volume through valves 1,2,3, and 4 until a certain pressure is reached, which then opens valves 8,9,10, and 11 to allow the CO2 concentration to be monitored by the NDIR CO2 analyzer.
-
Manometric Chamber Isolated
Once the CO2 concentration steadies out, valves 3 and 8 close to isolate the manometric chamber (in red in the diagram above). The air is allowed time to equilibrate to the oven temperature, and then the pressure of the sample air is measured by the high precision pressure gauge.
-
CO2 Trapped
Next, valves 8,12, and 13 are opened to allow the sample air to flow through two liquid nitrogen traps that cause CO2 and N2O along with any residual water vapor to freeze out on the side of the tubing. Liquid nitrogen is at a temperature of -196ºC (-321ºF), much colder than the alcohol bath in the H2O trap. The rest of the sample air (now without any CO2, N2O, or H2O) is pumped out into the room. This leaves CO2, N2O, and any remaining H2O as the only gases in the manometric system. This process typically takes about 40 minutes because the air needs to flow through the traps slow enough to allow CO2 and N2O to freeze out. The second trap is there to be extra safe that no CO2 is lost.
-
CO2 "Cryopumped" to 10mL Glass Volume
Now the CO2 and N2O needs to be separated from the frozen water and transferred over into the 10mL volume inside the oven. This is done by placing a mixture of alcohol and liquid nitrogen at a temperature of -65ºC (-85ºF) over the CO2 traps and placing pure liquid nitrogen over the 10mL volume inside the oven. This causes CO2 and N2O to turn back to gas in the CO2 traps but keeps water frozen. The liquid nitrogen cooling down the 10mL volume causes all the CO2 and N2O to flow from the CO2 traps into the 10mL volume and refreeze (a process called "cryopumping"). Once all the CO2 and N2O is trapped in the 10mL volume, valve 5 closes to isolate the pure CO2 and N2O.
-
CO2 Pressure Measured
The CO2 and N2O is allowed to thaw and equilibrate in the 10mL volume to the oven temperature, then the pressure in the 10mL volume is measured by the high precision pressure gauge.
-
CO2 Concentration Calculated
From the known volume ratio between the 6L and the 10mL glass volumes and the measured pressures in the initial 6L volume of air and the final 10mL volume of the CO2 and N2O fraction of air, the concentration of CO2 inside the Primary standard can be determined through mathematical calculations using Dalton's Law of Partial Pressures and a modified version of the Ideal Gas Law. You are probably wondering how the concentration of pure CO2 can be determined when there is still N2O in the final 10mL volume. In fact, since the concentration of N2O does not change much in our atmosphere and is measured precisely on a separate calibration instrument anyways, we just subtract it from our final calculation for CO2 concentration.
**If you want to learn more about the Manometer and how it works, check out the links below**

 Previous
Previous