Measuring & Analyzing Greenhouse Gases: Behind the Scenes
Where Do Standards Come From?
Every one of the standards are filled with clean air from a special place in the Colorado mountains called Niwot Ridge. Niwot Ridge is essentially the birth place of the very important standards that scientists rely on across the world.
When filled tanks come back from Niwot Ridge, they are put aside for at least a week to ensure that the air inside the tank is well-mixed. Then the new air tanks are ready to become calibrated standards in the Calibration Lab.
Why is Natural Air Used in Standards?
After many years of trial and error, we have learned that natural air is the BEST medium [The molecules surrounding the gases we are interested in] to create long-term standards of trace gases. Initially, we tried using synthetic mixtures starting with nitrogen (N2) and adding certain concentrations of the greenhouse gases to it. However, these synthetic air standards were not as stable as hoped. There are many interactions that different gases create when mixed together in natural air, which made measurements of synthetic air slightly different than those of natural air. The only way to accurately measure a greenhouse gas that resides at very small amounts in natural air is to use well-known standards of that same natural air.
Niwot Ridge
NOAA's Carbon Cycle Greenhouse Gases (CCGG) group uses Niwot Ridge, a high elevation mountain research site, to fill large air tanks (also called "cylinders") with clean air that are then used to calibrate greenhouse gas measurement equipment used by scientists all over the world. In fact the CCGG Calibration Lab, where the cylinders of air are very precisely measured after being filled at Niwot Ridge, has been designated the world's Central Calibration Laboratory (CCL) for greenhouse gases. It's vital that all the greenhouse gas measurement labs around the world are inter-compared to make sure that all labs get the same result from the reference materials [This is what the gas analyzers use to calibrate their measurements] such as these air tanks, also known as "standards." The CCGG group has been involved in maintaining greenhouse gas standards since the 1970's. Today the standards preparation facility at Niwot Ridge makes more than 400 standards per year, more than 5000 standards total, and plays a very important role in our ability to monitor greenhouse gases around the world .
View Larger Map
If you explore the area around the standards prep facility you will see there is very little human development around the facility, but if you zoom out far enough you will see the highly populated front range to the east of the mountains. This site was chosen for these reasons; it's close enough to the Central Calibration Lab in Boulder, and it's remote enough to provide clean air that is not impacted by urban pollution. Clean air is important because it is usually stable, meaning gases won't undergo unwanted reactions within the air tanks that will change their concentrations. In order to have a "standard," the concentrations of the gases within the tank must be constant over time.
The Filling Process
Step 1: Load up the Truck
Duane Kitzis is in charge of filling the standard air cylinders at Niwot Ridge, which is typically done once or twice a week depending on the need for new standards. The process starts at the CCGG labs in Boulder, and the first step is to load up the lab truck with empty air tanks that will be filled at Niwot Ridge.

Step 2: Travel to Standards Prep Facility
Next, Duane drives about 30 miles from the CCGG Boulder Central Facility to the standards prep facility in the mountains west of Boulder. The last couple miles of driving is on a rough dirt road that climbs up the mountain. The dirt road is sometimes blocked by large snowdrifts even into June and July, which makes it hard to reach the standards prep facility by truck. That's why a snowcat is used to transport the standard air tanks to and from the standards prep facility during the winter.

Step 3: Unload the Truck
At the standards prep facility, the empty air cylinders are unloaded across the ramp from the truck bed into the standards prep building. There are many times throughout the year when the road is impassable and Duane has to walk or ski up the road. That's why Duane brings extra cylinders whenever he can, and especially when he has helpers along (like the author of this article!). There is plenty of storage for these extra cylinders in an old trailer that used to be Duane's office until the wildlife decided otherwise.

Step 4: Capturing the Clean Air

To obtain the cleanest ambient air (the fill air), Duane collects and stores ambient air from early in the morning (12am to 5am) into ballast tanks using an automatic timer. Early in the morning, the air almost always flows from the undeveloped Rocky Mountains to the west, whereas later in the day the wind sometimes changes and brings air up from the populated and polluted Denver/Boulder region to the east (take another look at that Google map above). During the day, as other researchers go about their research, there can also be vehicle traffic up the dirt road that could contaminate the fill air.
The fill air is collected from 35 feet above the ground on a tower, an additional way to ensure that the air is nice and clean. The fill air is pumped and pressurized to 3,500 psi (that's 238 times atmospheric pressure at sea level!) in ballast tanks, like those normally used for scuba gear, using an oil-less pump. The pump must NOT have petroleum products contact the fill air because it would contaminate it with unwanted chemicals. The ballast tanks are pressurized so high because the new standards being filled at Niwot Ridge must be pressurized to around 2,000 psi (136 atmospheres), which means the ballast tanks need to be at a higher pressure to be able to push the fill air into the highly pressurized standard tank.
Ambient air always contains water vapor, but it is important to make sure the fill air is dry because unwanted reactions can occur at the high pressure inside the air tanks if water is present. As the fill air flows from the pump into the ballast tanks, it flows through two different “dryers” to ensure that most of the water is removed. First, a coalescent filter [Similar to a filter in a vacuum, the coalescent filter allows air to move through a fiber mesh without letting water droplets through. The water droplets collide (or coalesce) on the fiber mesh and fall down into the bottom of the filter where the water is eventually removed.] captures droplets of water that may have gone through the pump. Second, any water that is left is removed as the air flows through two stainless steel tubes about 20" long that are filled with Anhydrous Magnesium Perchlorate, which is a chemical compound that strongly absorbs any remaining water. It also dissolves in water, so the Anhydrous Magnesium Perchlorate tubes need to be replaced every month. It is impossible to remove every last molecule of water, but this double whammy gets very close. In fact there is less than 1 part per million (ppm) of water left afterward, which means for every 1 million molecules of air in the ballast tanks, only one molecule is water (H2O).
Step 5: Tank Filling Setup
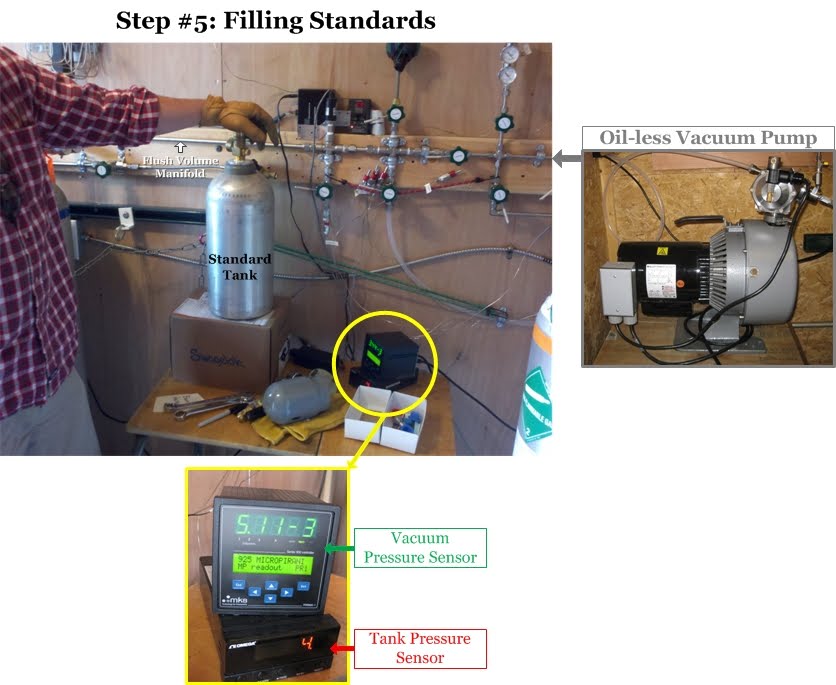
The tank filling process begins by connecting a new, empty air tank to the flush volume manifold [This is the "highway" of tubing that the fill air flows through to enter the air tank]. Another oil-free vacuum pump is used to remove all the air from the flush volume manifold and the tubing connecting the ballast, zeroed, and spiked tanks. Duane uses a digital pressure sensor (green in the photo below) to determine when the manifold is completely free of air. Then Duane opens the valves along the wall to allow the clean air from the ballast tanks to flow through the manifold into the new air tank. Another digital pressure sensor (red in the photo below) tells Duane the pressure (psi) inside the air tank, and once it reaches about 2,000 psi (136 atmospheres), the tank is full. Then he throws all that work away! This first filling of the air tank is just to get the tank used to the new air being pumped into it. The walls of a new air tank sometimes have unwanted gases clinging to them that will exchange with the new clean air being pumped in, thus increasing the concentrations. For some gases the opposite can happen: the cylinder walls will suck a little bit of the gas out of the clean air, thus reducing the concentration in the air. This all happens when air is first introduced into the air tank, and thus the first fill is thrown away after a few minutes. This ensures that when the final air is added to the soon-to-be standard, it will be stable through time.
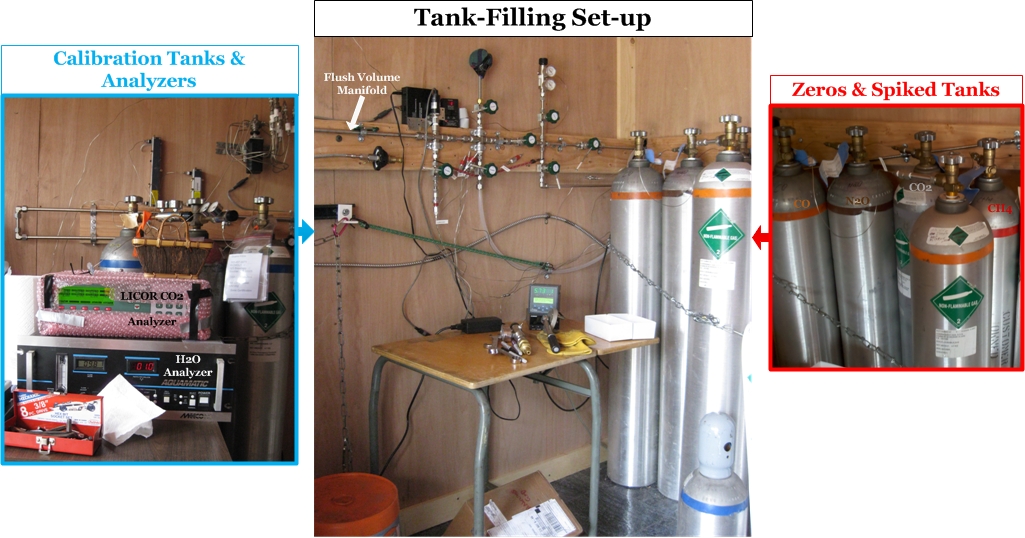
Step 6: Filling Standards
Duane makes up the cylinders according to the requests of CCGG and other researchers around the world asking him for standards with specific “target” concentrations of trace gases. “Targeting” is the tricky part, getting a certain concentration of CO2, CH4, or some other greenhouse gas in the cylinder by using outside air that already has some concentration of these gases. The target values are not exact, the exact amount of each gas in each tank will be determined later in the Calibration Lab, but the final tank concentrations need to be within a small range requested for each standard. Typically Duane can target the concentrations of individual gases to within 2 ppm. Duane uses "zero" [Zero air tanks (or zeros) have had a certain gas removed from them, or at least enough removed that the concentration is very low compared to what we see in the normal atmosphere.] and "spiked" [Spiked air tanks contain air with MUCH HIGHER concentrations of a trace gas that is not seen in the normal atmosphere.] air tanks to target gases within the standards he creates. Typically researchers request standards with low, medium, and high concentrations of the trace gas they are interested in. The "medium" standard is typically filled with the clean ambient air stored in the ballast tanks, which generally represents the background concentrations [The concentrations of trace gases higher in the atmosphere that have been well-mixed over time and represent clean, unpolluted air.] of trace gases for the continental US. But, the high and low standards require more thinking because Duane needs to use the spiked or zero tanks to raise or lower the trace gas concentrations in the new air tank from the ambient air values captured at Niwot Ridge.
- Lower than ambient concentration - First a certain percentage of the tank is filled with air from the zero tank, which has little or no concentration of the trace gas, then the rest of the tank is filled with the ambient fill air. If half of the tank is filled with the zero and the other half filled with the ambient air, then the concentration of the trace gas of interest will be half what it was in the ambient fill air.
- Higher than ambient concentration - First the flush volume manifold is filled with a calculated amount of pressure from one of the spiked air tanks, then the ambient fill air flushes that spiked air into the tank until the standard tank is pumped up to the desired pressure (typically 2,000 psi).
The filled cylinders, each with it’s own target mixture, are now ready to be brought down the mountain to the CCGG Boulder Central Facility where they will be measured very precisely in the Calibration Lab to find out exactly what concentrations of all the greenhouse gases ended up in the new standard. Duane now gets to find out whether he hit his targets or not. If he got them wrong one day, it’ll be ok. He’ll just wait until someone sends in a request for the concentrations that he did make, but he will have to head back up to Niwot Ridge and have another go to get the right concentrations for the order.
If you want to learn more about the standard preparation facility and standards, check out the link below:

 Previous
Previous