What are Halons?
Halons are used primarily as fire extinguishing agents, both in built-in systems and in portable fire extinguishers. There have been some reported uses as tracers for studies of circulation both in the atmosphere and in buildings. All halons contain bromine which is 40-100 times more effective at destroying ozone than chlorine. Synergistic interactions between bromine, derived mainly from the halons and methyl bromide, and chlorine in the stratosphere are responsible for 30-40% of the Antarctic ozone hole.
The Montreal Protocol on Substances That Deplete the Ozone Layer and its subsequent amendments called for the elimination of production on January 1, 1994. The most commonly used halons are halon-1211 (CBrClF2), halon-1301 (CBrF3), and halon-2402 (C2Br2F4). The numbering system for the halons is different but more simple than for that of the CFCs. The first digit from the left is for the total number of carbon atoms, second is for the number of flourine atoms, third is for the number of chlorine atoms, and last is the number of bromine atoms.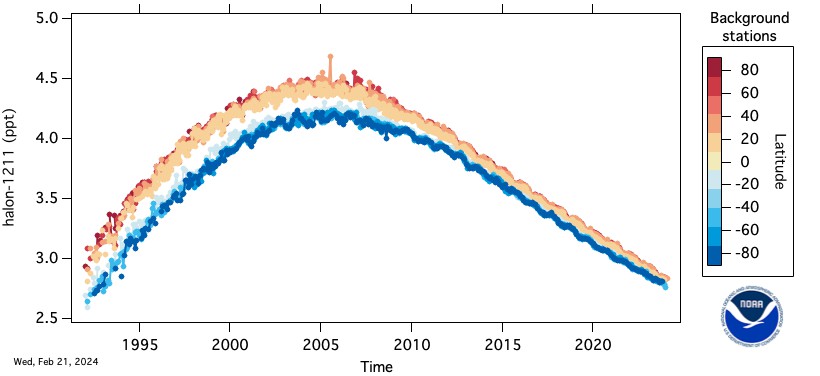
Halon-1211 is used primarily in portable fire extinguishing systems in the Western Hemisphere and Western Europe. Halon-1301 is used in flooding systems for fire and explosive containment. Halon-2402 is used as a fire extinguishing agent in Japan, Russia, China, and the former states of the Soviet Union. At the present, there are no known drop-in replacements for the halons, although many groups are working on potential replacements.
The HATS group of GML measures the halons in the atmosphere by electron capture-gas chromatography (EC-GC) and gas chromatography-mass spectroscopy (GC-MS). Sub-samples of 100-250 cc are taken from the flasks of air collected routinely at remote sites around the world and analyzed in Boulder, CO. Halon-1211 is also measured by the airborne ACATS project and by the CATS in situ program.
