Frequently Asked Questions
Categories
Questions about Greenhouse Gases:
- What is the carbon cycle?
- How much has the amount of CO2 in the atmosphere changed in the past?
- How do we know that humans are responsible?
- I simply cannot believe that humans can have much influence on the vastness of the earth.
- Can we avoid increasing climate change without dramatically lowering atmospheric CO2 emissions from fossil fuel burning?
- What is the greenhouse effect?
- How can minor atmospheric gases have such a large impact on climate?
- Which major greenhouse gases does GML measure?
- What are isotopes of greenhouse gases?
- How are the amounts of greenhouse gases measured?
- Why are isotopes of greenhouse gases important?
- Where are these gases measured?
- How much CO2 is in the atmosphere?
- How do we know CO2 in the atmosphere is increasing?
- How much has the amount of CO2 in the atmosphere changed in history?
- I've heard more CO2 in the air could be beneficial for plants and crops.
What is the carbon cycle?
 The Carbon Cycle is a complex series of processes through which all of the carbon atoms on earth rotate. The same carbon atoms in your body today have been used in countless other molecules since time began. The wood burned just a few years ago could have produced carbon dioxide
which through photosynthesis became part of another plant. When you eat that plant, the same carbon from the wood which was burnt can become part of you. The carbon cycle is the great natural recycler of carbon atoms. Carbon forms the backbone of organic molecules from which all
forms of life that we know have been built. In fact, it is (micro-)organisms that control most of the recycling of carbon and other elements on earth. Because organisms are affected by climate, the latter has an influence on the recycling, while some of the molecules involved in the recycling, such as carbon dioxide and methane, influence the climate through the greenhouse effect.
The Carbon Cycle is a complex series of processes through which all of the carbon atoms on earth rotate. The same carbon atoms in your body today have been used in countless other molecules since time began. The wood burned just a few years ago could have produced carbon dioxide
which through photosynthesis became part of another plant. When you eat that plant, the same carbon from the wood which was burnt can become part of you. The carbon cycle is the great natural recycler of carbon atoms. Carbon forms the backbone of organic molecules from which all
forms of life that we know have been built. In fact, it is (micro-)organisms that control most of the recycling of carbon and other elements on earth. Because organisms are affected by climate, the latter has an influence on the recycling, while some of the molecules involved in the recycling, such as carbon dioxide and methane, influence the climate through the greenhouse effect.
I simply cannot believe that humans can have much influence on the vastness of the earth.
The influence of each of us individually is minuscule indeed, and many of us instinctively have this reaction when we first hear about climate change. But think again. There are 6.5 billion of us, and our collective influence is clearly visible. When we fly over the Midwest of the United States we see that a vast landscape has been completely transformed. What was once prairie has now been divided into neat blocks, and we determine what grows in every one of those blocks, and pretty much also what grows outside of them. We have made artificial lakes, built huge cities, often with very visible air pollution haze, and railways and roads. We have leveled mountains in mining operations. Many of the world's major fisheries are in decline. We have introduced large quantities of new chemicals into the environment, often with unknown effects. The evidence is solid that we have changed the composition of our atmosphere. If we take a good look around us, we shouldn't be too surprised that we might also be capable of influencing the earth's climate, even if unintentionally.
How do we know that humans are responsible?
The evidence for a dominating human role in the CO2 increase is extremely strong. The 38% increase (in 2009) in atmospheric CO2 observed since pre-industrial times cannot be explained by natural causes. CO2 levels in the atmosphere have varied naturally throughout Earth's history. However, CO2 levels are now higher than any seen in the past 800,000 years. When we add the observed CO2 increase in the atmosphere to the observed increase in the oceans, the sum is approximately equal to all of the coal, oil, and natural gas burned since the 19th century. Furthermore, the observed progressive depletion in carbon-13 (see the question below about isotopes) shows that the source of the CO2 is either fossil fuels or deforestation because both produce CO2 depleted in carbon-13. The atmospheric CO2 increase cannot have come from the oceans because that would not have caused any depletion of carbon-13. In fact, carbon in the oceans has itself become gradually depleted in carbon-13, with the greatest depletion at the surface. That implies that the signal is imposed from the atmosphere. The next piece of evidence is that we also observe a depletion of radioactive carbon-14 in the atmosphere and oceans, with the strongest signal in the atmosphere suggesting it is the place where the depletion originates. Fossil fuels contain no carbon-14, and their combustion produces CO2 without carbon-14. Deforestation does not cause a change in atmospheric carbon-14. Finally, the annual mean CO2 abundance in the northern hemisphere is higher than in the southern hemisphere, and more so in recent years compared to the early years of atmospheric CO2 measurements. This suggests a growing source of CO2 in the northern hemisphere, which is in fact where most of the fossil fuel burning takes place.
How much has the amount of CO2 in the atmosphere changed in the past?
Ice cores have been collected from Antarctica and Greenland which contain information stored in the ice that can be used to reconstruct climates thousands of years ago. As snow accumulates on ice caps and ice sheets where temperatures usually remain below freezing year round, it lays down a record of the environmental conditions at the time of its formation. Over time the snow, buried under further accumulations, is compacted to ice, preserving the climatic information. Air bubbles trapped in the ice can be analyzed to reconstruct the atmospheric composition at the time when the ice formed. Measurements of the amount of greenhouse gases in these bubbles show that the "pre-industrial" amount of CO2 in the atmosphere was about 280 parts per million (ppm), about 100 ppm below today's (2006) value. The figure below show results of CO2 measurements of air trapped in ice cores taken at the Law Dome site in Antarctica, along with present day measurements at the GML Mauna Loa Observatory in Hawaii. CO2 amounts have increased about 36% in the last 150 years, about half of that in the last three decades.
Can we avoid increasing climate change without dramatically lowering atmospheric CO2 emissions from fossil fuel burning?
The answer is essentially no. If we decrease emissions of greenhouse gases like methane, nitrous oxide, or chlorofluorocarbons, their atmospheric burden, and thus their climate forcing, will decrease in one to several decades. However, the effect of CO2 emissions on the forcing of climate change is primarily dependent on the total amount emitted since the beginning of the industrial revolution. Roughly speaking, as long as we continue to emit CO2, its climate forcing will continue to increase, so that as time progresses its effect on climate forcing would gradually overwhelm the others more and more. Once CO2 has been emitted into the atmosphere, the carbon cycle will redistribute it between the atmosphere, oceans, and terrestrial biosphere, but it will not disappear from those systems for thousands of years. This fact explains why all future scenarios holding atmospheric CO2 constant at some specified level need to drive emissions gradually to zero. This will occur sooner if the level is set at 500 ppm than if it is set at 600 ppm. However, there have been proposals to mitigate climate change not by decreasing greenhouse gas emissions, but by increasing the reflection of incoming solar radiation with mirrors, aerosols (small particles), or other means. Increasing atmospheric CO2 is inextricably linked with increasing acidity of the oceans. The acidification has already been measured, and if the increasing CO2 trend continues it would come to pose a serious extinction threat to major classes of marine organisms, including corals.
What is the greenhouse effect?
The Sun, which is the Earth's only external form of heat, emits solar radiation mainly in the form of shortwave visible and ultraviolet (UV) energy. As this radiation travels toward the Earth, the atmosphere absorbs about 25% of it, and about 25% is reflected by the clouds back into space. The remaining radiation travels unimpeded to the Earth and warms its surface. The Earth releases back to space the same amount of energy it has absorbed from the Sun. However, the Earth is much cooler than the Sun, so the energy re-emitted from the Earth's surface is much weaker, in the form of invisible longwave infrared (IR) radiation, sometimes called heat radiation. If you stand close to a hot object, but do not touch it, you can feel how the IR radiation heats your skin, although you cannot see the IR rays.
Gases that absorb and trap this IR radiation, such as water vapor (H2O), carbon dioxide (CO2), methane (CH4) and nitrous oxide (N2O) are known as "greenhouse gases". The atmosphere acts like the glass in a greenhouse, allowing much of the shortwave solar radiation to travel through unimpeded, but trapping a lot of the longwave heat energy trying to escape back to space. This process makes the temperature rise in the atmosphere just as it does in the greenhouse. This is the Earth's natural greenhouse effect and keeps the Earth 33 °C warmer than it would be without an atmosphere, at an average 15 °C (59° F).
How can minor atmospheric gases have such a large impact on climate?
The major gases, nitrogen (N2), oxygen (O2), and argon (Ar), which together comprise about 99.8% of the atmosphere, do not absorb visible light, nor infrared light. If the atmosphere contained only those three gases, the radiation would go right through without any effect on the heating of the atmosphere or surface. That leaves it to the minor gases such as water vapor, carbon dioxide, methane, nitrous oxide, ozone, and others to absorb infrared light. The total mass of the atmosphere is very large, about 5 x 1021 grams, or 5 million times a billion metric ton. The amounts of the minor gases are therefore still very large, sufficient to cause the absorption of a major fraction of infrared light in the atmosphere.
Which major greenhouse gases does GML measure?
|
Carbon Dioxide (CO2) is a colorless, odorless gas consisting of molecules of two oxygen atoms and one carbon atom. Carbon dioxide is produced when an organic carbon compound (such as wood) or fossilized organic matter (such as coal, oil, or natural gas) is burned in an excess of oxygen. Carbon dioxide is removed from the atmosphere by carbon dioxide "sinks" such as absorption by seawater, and photosynthesis by ocean-dwelling plankton and land plants, including forests and grasslands. However, seawater also releases CO2 to the atmosphere, as do land plants and soils when CO2 is released as respiration. |

|
Methane (CH4) is a colorless, odorless non-toxic gas consisting of molecules of four hydrogen atoms and one carbon atom. Methane is combustible, and mixtures of about 5 to 15% in air are explosive. It is the main constituent of natural gas, a fossil fuel. It is released when organic matter decomposes in environments lacking sufficient oxygen. Natural sources include wetlands, swamps and marshes, termites, and oceans. Man-made sources include the mining and burning of fossil fuels, digestive processes in ruminant animals such as cattle, rice paddies and the buried waste in landfills. Most methane is broken down in the atmosphere by reacting with small very reactive molecules called hydroxyl (OH) radicals. |

|
Nitrous oxide (N2O) is a colorless, non-flammable gas with a sweetish odour, commonly known as "laughing gas", and sometimes used as an anaesthetic. Nitrous oxide is naturally produced in the oceans and in rainforests. Man-made sources of nitrous oxide include nylon and nitric acid production, the use of fertilizers in agriculture, cars with catalytic converters and the burning of organic matter. Nitrous oxide is broken down in the atmosphere by chemical reactions that involve sunlight. |
|
Sulfur hexafluoride (SF6) is an extremely potent greenhouse gas. SF6 is very persistent, with an atmospheric lifetime of more than a thousand years. Thus, a relatively small amount of SF6 can have a significant impact on global climate change. The primary user of SF6 is the electric power industry. Because of its inertness and dielectric properties, it is the industry's preferred gas for electrical insulation, current interruption, and arc quenching (to prevent fires) in the transmission and distribution of electricity. SF6 is used extensively in circuit breakers, gas-insulated substations, and switchgear. |
GML also measures the common isotopes of CO2 and CH4, in collaboration with the Stable Isotope Laboratory at the University of Colorado.
What are isotopes of greenhouse gases?
The nuclei of atoms are composed of protons and neutrons. Protons are electrically charged and their number determines the chemical character of the atom, or its place in the periodic table of the elements. The same chemical element can have a nucleus with a slightly different number of neutrons, which changes its chemical character only very slightly, but makes it either heavier or lighter because the mass of each atom is almost totally determined by the mass of its protons and neutrons. Isotopes of interest in the carbon cycle are carbon-12, with 6 protons and 6 neutrons, which makes up about 98.9% of all carbon, and carbon-13, with 6 protons and 7 neutrons, making up about 1.1%. Also of interest is carbon-14, with 6 protons and 8 neutrons, which is radioactive and has extremely low abundance. Oxygen isotopes of interest are oxygen-16 (8 protons and 8 neutrons), the most common, and oxygen-18 (8 protons and 10 neutrons). In the case of CO2, we can have molecules composed of carbon-12 with two oxygen-16 atoms, the most common, or other combinations such as carbon-13 with two oxygen-16s, or carbon-12 with one oxygen-16 and one oxygen-18, etc. The abundance ratios of the various combinations can be measured extremely accurately.
Why are isotopes of greenhouse gases important?
Chemical and biological processes in nature, such as respiration, photosynthesis and atmospheric chemical reactions, often show very slight preferences for one isotope over another. For example, photosynthesis discriminates against the heavy 13C isotope, and plant matter and respired CO2 is therefore depleted in 13C relative to the atmosphere. Careful analysis of the isotopic composition of atmospheric trace gases can provide valuable information on the sources and sinks of the gases concerned because each natural process leaves its isotopic "signature" in the gases it produces.
How are the amounts of greenhouse gases measured?
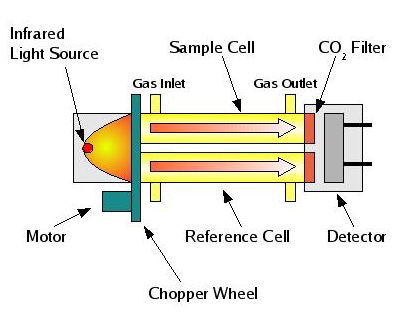
Non-isotopic measurements of greenhouse gases are generally made with two techniques: Non-Dispersive Infrared (NDIR) analysis, or by gas chromatography.
The amount of CO2 in the atmosphere is large enough that the NDIR method of measurement works well. An NDIR analyzer relies on the same principle of IR absorption that makes greenhouse gases important in the first place. An infrared analyzer consists of an infrared source at one end, and an infrared detector separated by a gas cell. The gas of interest is passed through this cell, and absorbs some of the infrared radiation coming from the source. The detector converts the amount of IR reaching it to a usable signal, such as a voltage. So as the concentration of CO2 changes in the sample, the signal from the detector changes. By flowing a gas with a known amount of CO2 through the cell, we can calibrate the analyzer so that the voltage output from the detector can be converted into amounts of CO2.
Gas Chromatography is a separation method in which gas mixtures flow over a material that retains some components more than others, so different components flow over the material at different speeds. The gas is separated into individual chemical components which can then pass through a detector, which varies in output for each component in the mixture. This is called a chromatogram. By measuring the height of each peak in a chromatogram, the amount of a component (such as CH4) can be calculated.
Chromatography is the preferred method for gases other than CO2 since it is well suited for small samples with small amounts of the gas of interest.
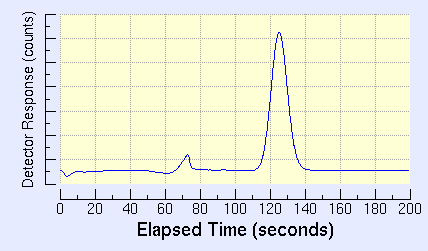
Example chromatogram showing a methane (CH4) peak.
Where are these gases measured?
GML's Carbon Cycle group (CCGG) makes continuous and discrete measurements of greenhouse gases at numerous worldwide surface sites, towers, aircraft, and ships of opportunity. There is a world map of the many different sampling sites. Air samples are collected in glass flasks from sampling sites of the NOAA GML CCGG Cooperative Air Sampling Network and returned to the CCGG laboratory in Boulder, Colorado for analysis. In-Situ, continuous measurements are made at the 4 baseline GML Observatories, and at 2 tall tower sites in the United States.
How much CO2 is in the atmosphere?
For the year 2006, the estimated global average CO2 amount was about 381 parts per million (ppm). If we use a value of 5.13 ×1018 kg for the mass of the dry atmosphere (excluding water vapor), and take into account that the average molecular weight of air equals 29.0 g/mole and the atomic weight of carbon 12.0 g/mole, then 1 ppm of CO2 corresponds to 2.12 Gt (billion metric ton) of carbon. This gives us a value of about 808 Gton of carbon in the atmosphere. If we include the mass of the two oxygen atoms in each CO2 molecule, the total mass of CO2 would be 2960 Gton. Watch out: in the news and in reports you may see emissions expressed either as tons of carbon or as tons of carbon dioxide. The latter is 3.67 times heavier than the former, although the same number of CO2 molecules is involved.
How do we know CO2 in the atmosphere is increasing?
Very carefully calibrated measurements have confirmed that CO2 is increasing in the atmosphere and that human activities are the primary cause. CO2 measurements, started by Charles D. Keeling of the Scripps Institution of Oceanography, have been taken directly from the atmosphere over the last ~50 years. CO2 trends for earlier times have been derived from measurements of CO2 in air trapped in bubbles in polar ice and in mountain glaciers.
How much has the amount of CO2 in the atmosphere changed in history?
Ice cores have been collected from Antarctica and Greenland which contain information stored in the ice that can be used to reconstruct climates thousands of years ago. As snow accumulates on ice caps and sheets where temperatures usually remain below freezing year round, it lays down a record of the environmental conditions at the time of its formation. Over time the snow, buried under further accumulations, is compacted to ice, preserving the climatic information. Air bubbles trapped in the ice can be analyzed to reconstruct the atmospheric composition at the time when the ice formed.
Measurements of the amount of greenhouse gases in these bubbles show that the "pre-industrial" amount of CO2 in the atmosphere was about 280 parts per million (ppm), about 100 ppm below todays value. The figure below show results of CO2 measurements of air trapped in ice cores taken at the Law Dome site in Antarctica, along with present day measurements at the GML Mauna Loa Observatory in Hawaii. CO2 amounts have increased about 36% in the last 200 years.
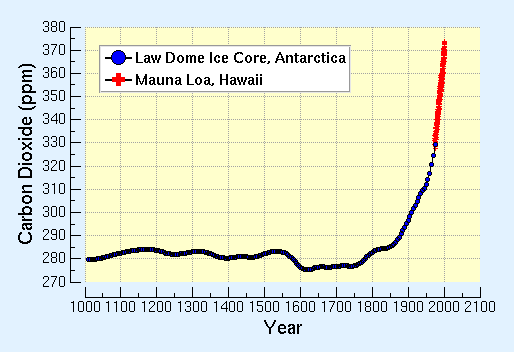
Law Dome Ice Core data source:
D.M. Etheridge, L.P. Steele, R.L. Langenfelds,
R.J. Francey, J.-M. Barnola and V.I. Morgan. 1998.
Historical CO2 records from the Law Dome
DE08, DE08-2, and DSS ice cores. In
Trends: A Compendium of Data on Global Change.
Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory,
U.S. Department of Energy, Oak Ridge, Tenn., U.S.A
I've heard more CO2 in the air could be beneficial for plants and crops.
The impacts of climate change on crops and natural ecosystems depend on complex interactions among increased CO2, rising temperatures, water and nutrient availability, wildfires, plant diseases and pest outbreaks. Elevated levels of CO2 can essentially fertilize plants and crops. However, plant growth is also affected by other factors in addition to CO2 ? factors that will be influenced by climate change. Modest temperature increases, for example, can enhance growth, but if temperatures increase too much, growth actually declines. Rising temperatures also increase the process by which plants release CO2. Higher temperatures can increase the rate of evaporation, drying out soils. Insufficient water decreases plant growth. Plants also cannot respond to more CO2 unless sufficient nutrients are available. Furthermore, the growth-enhancing effects of CO2 may diminish over time. Real-world crop yields would also be subject to the hazards of droughts and floods under a changing climate.
