More than two years after road access and electrical power to the Mauna Loa Observatory was cut off by lava flows, NOAA staff continue to make critical measurements of the atmosphere and other environmental variables at the remote site.
In 2023, observatory staff installed solar panels at the site and resumed some measurements, including the independent carbon dioxide monitoring programs run by the Global Monitoring Laboratory and Scripps Institution of Oceanography, as well as other atmospheric measurements.
Construction of a temporary road to access the observatory site is anticipated to begin in summer 2025.
Media can contact: Theo Stein (303) 819-7409 (theo.stein@noaa.gov)
Organization(s):
 State University of New York (SUNY)
State University of New York (SUNY)
 State University of New York (SUNY) / Marine Sciences Research Center (MSRC)
State University of New York (SUNY) / Marine Sciences Research Center (MSRC)
What does this program measure?
This project measures 14CO, a naturally occurring radioactive tracer, in units of molecules/cm3. It also measures 13CO, C18O. Also noted are local meteorological conditions during sampling (wind speed, direction, temperature, precipitation).
How does this program work?
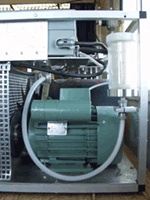
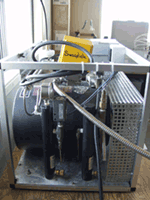
We collect large, whole air samples, extract the air in the laboratory, isolate the gas, and measure it using accelerator mass spectrometry. Samples are collected weekly, during down slope conditions (around 4am local time). Local meteorological conditions during sampling are noted (wind speed, direction, temperature, precipitation). The system is automated and the cylinder is filled to approximately 800 psi.
MLO observers note the sample pressure, close the sample cylinder, ship the cylinder to Stony Brook, and then attach a new sample cylinder to the sampling system and replace the drying cartridge. The night prior to collection, the valve to the sample cylinder is opened. Instrumentation consists of sample cylinder and National Weather Service Meteorology instruments.
Why is this research important?
We use 14CO as a tracer of OH (hydroxyl) concentration, which is important for its reactivity with many other trace gases. By doing so, we can determine the lifetime of those trace gases, and whether or not the lifetime is changing.
Are there any trends in the data?
To be determined.
How does this program fit into the big picture?
What is it's role in global climate change?
OH radical (hydroxyl) is the most reactive oxidant in the atmosphere. Any variation in OH abundance will lead to changes in the abundances of many trace gas species in the atmosphere, including methane (CH4), carbon monoxide (CO), and volatile organic compounds.
Comments and References
DISCLAIMER:
This material is based upon work supported by the National Science Foundation. Any opinions, findings, and conclusions or recommendations expressed in this material are the opinions of the author(s) and do not necessarily reflect the views of the National Science Foundation.
Lead Investigator(s):
John E. Mak
631-632-8673
MLO Contact(s):
David Nardini
808-933-6965 (x229)
Paul Fukumura
808-933-6965 (x223)
Web Site(s)
http://atmos.msrc.sunysb.edu/npages/Faculty_Prof/Mak.html
Date Started
October, 1998
(inactive 10/01-08/03)
Related Programs
NSF Globe Sunphotometer
Meteorology
Greenhouse Gases