RITS In Situ Monitoring Program
Overview
 The Radiatively Important Trace Species (RITS) program was the original gas chromatographs
used to make in-situ measurements at several field sites from 1983-2001. These GC's were
replaced by the current CATS Gas Chromatographs at the in-situ sampling sites.
The Radiatively Important Trace Species (RITS) program was the original gas chromatographs
used to make in-situ measurements at several field sites from 1983-2001. These GC's were
replaced by the current CATS Gas Chromatographs at the in-situ sampling sites.
The last of the RITS systems at the field sites was retired in 2001. An effort to finalize the 15 year RITS data set by checking the existing data base for errors and recovering information from problem periods is described in the RIts Data Recovery page.
Personnel
Thayne Thompson was the RITS project leader and is now retired. Geoff Dutton is the CATS project manager acting as the station liaison, managing equipment deployment and maintenance, and doing the data processing and quality control. David Nance is the RITS data manager.
Instrument History
- Dec 1983
- 1 channel manual gas chromatograph at South Pole, Antarctica
- (N2O, CFC-12, CFC-11)
- Jun 1986
- 2 channel RITS automated gas chromatograph at American Samoa
- (N2O, CFC-12, CFC-11, CFC-113, methyl chloroform, carbon tetrachloride)
- Oct 1986
- 2 channel RITS automated gas chromatograph at Barrow, Alaska
- (N2O, CFC-12, CFC-11, CFC-113, methyl chloroform, carbon tetrachloride)
- Jun 1987
- 3 channel RITS automated gas chromatograph at Mauna Loa, Hawaii
- (same as two channel except a higher precision N2O channel is added)
- additional channel added to American Samoa
- (high precision N2O)
- Aug 1987
- additional channel added to Barrow, Alaska
- (high precision N2O)
- Feb 1988
- 2 channel RITS automated gas chromatograph at South Pole, Antarctica
- (N2O, CFC-12, CFC-11, CFC-113, methyl chloroform, carbon tetrachloride)
- Oct 1994
- 4 channel automated CATS gas chromatograph at WITN Tower, Grifton, North Carolina
- Jun 1995
- 2 channel automated CATS gas chromatograph at WLEF Tower, Parkfalls, Wisconsin
- Jul 1995
- 4 channel automated CATS gas chromatograph at Harvard Forest, Massachusetts
- Aug 1995
- 1 channel automated CATS gas chromatograph at Alert, Canada
- (N2O)
- Jan 1998
- 4 channel automated CATS gas chromatograph at South Pole, Antarctica
- (N2O, SF6, CFC-12, Halon-1211, CFC-11, CFC-113, chloroform, methyl chloroform, carbon tetrachloride, HCFC-22, CH3Cl, and CH3Br)
- Jun 1998
- 4 channel automated CATS gas chromatograph at Barrow, Alaska
- (additionally measures HCFC-142b and OCS)
- Sep 1998
- 4 channel automated CATS gas chromatograph at Mauna Loa, Hawaii
- Dec 1998
- 4 channel automated CATS gas chromatograph at American Samoa
- Feb 1999
- RITS gas chromatograph at Barrow, Alaska retired
- Apr 2000
- 2 channels of RITS gas chromatograph at Mauna Loa, Hawaii retired
- 2 channels of RITS gas chromatograph at American Samoa retired
- Sep 2000
- final channel of RITS gas chromatograph at American Samoa retired
- Oct 2000
- 4 channel automated CATS gas chromatograph at Niwot Ridge, Colorado
- Nov 2000
- RITS gas chromatograph at South Pole, Antarctica retired
- Dec 2000
- final channel of RITS gas chromatograph at Mauna Loa, Hawaii retired
- Aug 2001
- RITS gas chromatograph at Niwot Ridge, Colorado retired
- Oct 2001
- 2 channel automated gas chromatograph at Ushuaia, Argentina.
- (N2O, SF6, CFC-12, CFC-11, CFC-113, methyl chloroform, carbon tetrachloride)
Analytical Technique
The RITS GC's made measurements of nitrous oxide (N2O), the chlorofluorocarbons: CFC-12 (CCl2F2), CFC-11 (CCl3F), and CFC-113 (CCl2F-CClF2) and the chlorinated solvents: methyl chloroform (CH3CCl3) and carbon tetrachloride (CCl4) once an hour.
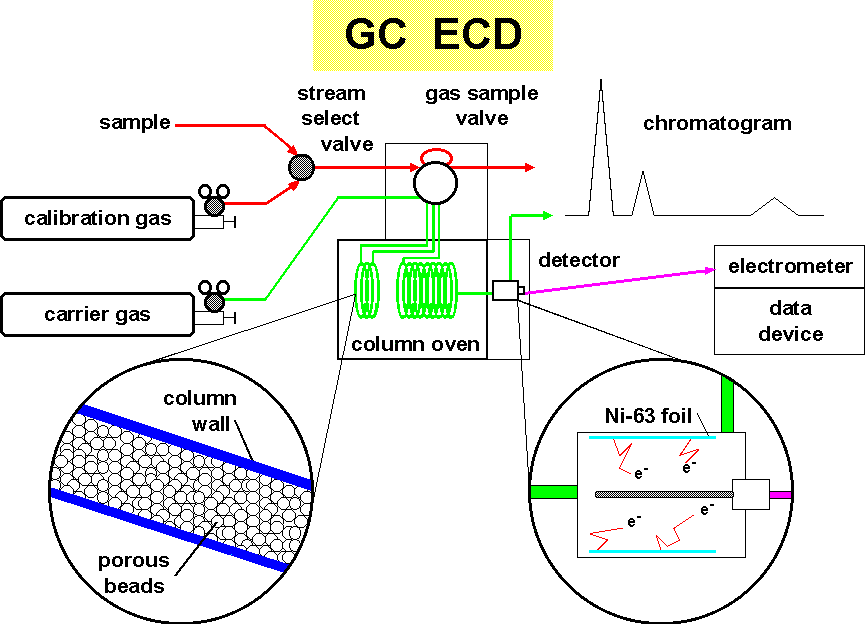
Air is drawn through a sampling line using a clean pump. This air flushes the gas sampling valve for about 5 minutes. The flushing is then stopped and the sample bleeds down to ambient pressure. The sample is inserted into a stream of inert carrier gas (usually nitrogen or a mixture of argon/methane) which pushes the air through the separation process.
The separation of the sample air is accomplished using gas chromatography. Tiny porous beads, sometimes coated with a liquid, interact with the mixture of molecules impeding there movement either because of their size or their solubility. In our case, this separation column is divided into two parts. When the chemicals of interest move onto the second column, the first column's flow is reversed to clean heavier compounds off before the next injection. This is called "backflushing".
Detection of the halocompounds is by an electron capture detector. A radioactive foil of nickel-63 is inside the pin-in-cup detector. The beta decay (an electron) ionizes the carrier gas forming an electron cloud. Periodically a large positive pulse is applied to the center electrode. This causes the free electrons to move to the electrode where they are measured as a tiny current. When a molecule containing halogen atoms, which has the property of enhanced affinity for electrons, enters the detector, it readily attracts and holds a free electron. The background current is thus reduced. In due time the electron capture molecules are flushed out of the detector and the current returns to its previous level.
The measure of the dip in the current curve is a measure of the amount of chemical present. By periodically injecting gas mixtures containing known quantities of the chemical of interest, calibration of the detector is accomplished.
Recording of the inverted current produces a curve called a "chromatogram".
